
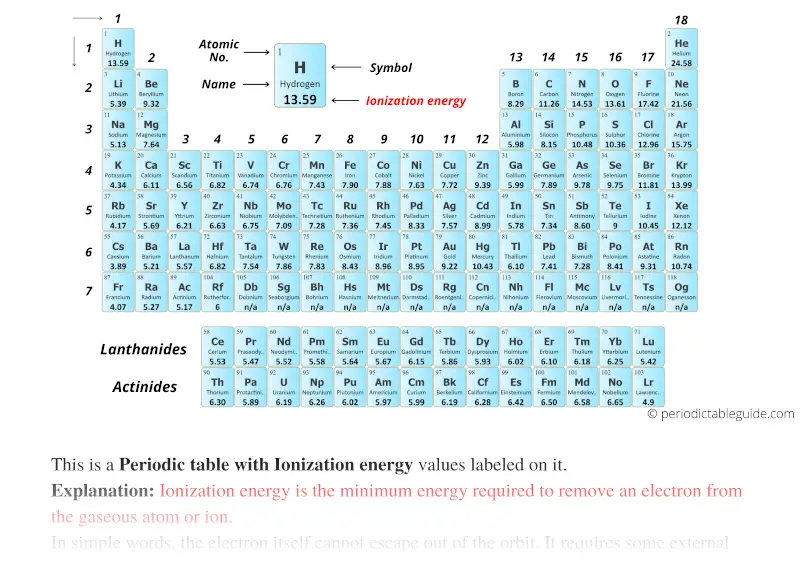
Predicting the ionization energy would be difficult with this alone. The effective nuclear charge is 7, the same as fluorine. What would be the effective nuclear charge for Cl? Would you predict the ionization energy to be higher or lower than fluorine?Ĭhlorine has the electronic configuration of 3 s 23 p 5 Overall the general trend for ionization energy is shown below. Notice how as the effective nuclear charge increases, so does the ionization energy. The effective nuclear charge is the difference between the total charge in the nucleus (the number of protons) and the number of shielded electrons. The electrons that are shielding the nuclear charge are the core electrons, or in the case of Period 2, the 1 s 2 electrons.

Table 10.9: Effective Nuclear Charge for Period 2 Main Group Elements Table 10.9 shows the effective nuclear charge along with the ionization energy for the elements in Period 2. When we look at the data in Table 10.7 again, we can see how the effective nuclear charge increases going across a period. Remember, the nuclear charge was used to describe why the atomic size decreased going across a period. The effective nuclear charge is the charge experienced by a specific electron within an atom. The second factor is that the effective nuclear charge increases. One factor is that the atomic size decreases. So if we look at the ionization energy trend in the Periodic Table and add it to the trend that exists with atomic size we can show the following on our Periodic Table chart.īut why does the ionization energy increase going across a period and decrease going down a group? It has to do with two factors. The Charge on the Nucleus Increases and Size Decreases Since there is an imbalance of positive and negative charges when a second electron is being removed, the energy required for the second ionization ( IE 2) will be greater than the energy required for the first ionization ( IE 1). In order to remove this electron, energy must be added to the system. Lithium has one electron in its outermost energy level. Lithium has an electron configuration of 1 s 22 s 1. Ionization Energy is the Energy Required to Remove an Electron
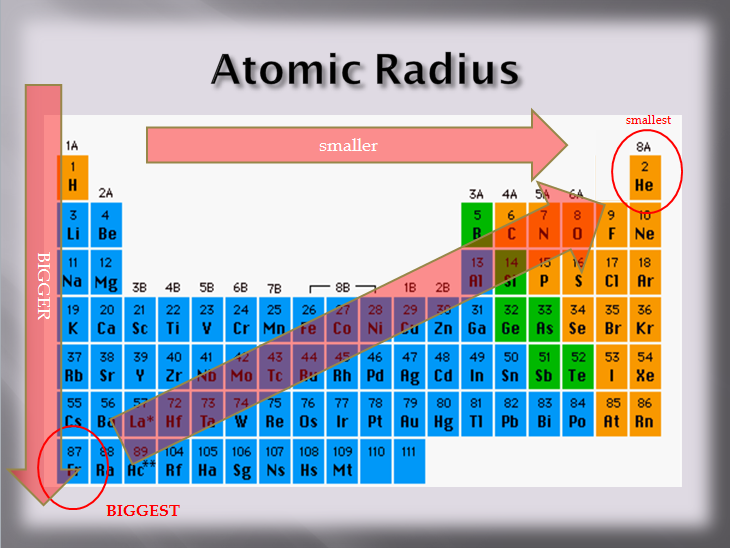
3 The Charge on the Nucleus Increases and Size Decreases.2 Ionization Energy is the Energy Required to Remove an Electron.


 0 kommentar(er)
0 kommentar(er)
